Appendix A - Glossary | NDT Technology for Quality Assurance of HMA Pavement Construction |The National Academies Press

Part III - Data Interpretation and Application | Supporting Materials for NCHRP Report 626 |The National Academies Press

Chapter 2 - Materials Testing for Construction Quality Determination | NDT Technology for Quality Assurance of HMA Pavement Construction |The National Academies Press

Chapter 2 - Materials Testing for Construction Quality Determination | NDT Technology for Quality Assurance of HMA Pavement Construction |The National Academies Press
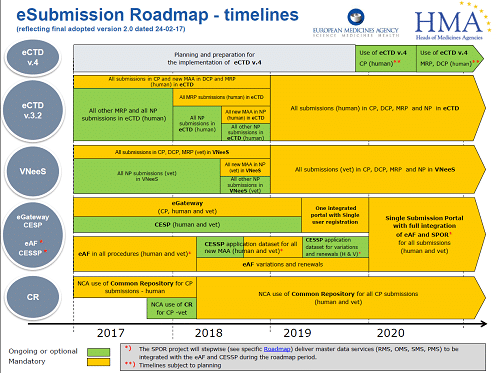
Practical guidance for procedures related to Brexit for medicinal products for human use approved via MRP/DCP

Appendix A - Glossary | NDT Technology for Quality Assurance of HMA Pavement Construction |The National Academies Press
European Medicines Agency pre-authorisation procedural advice for users of the centralised procedure