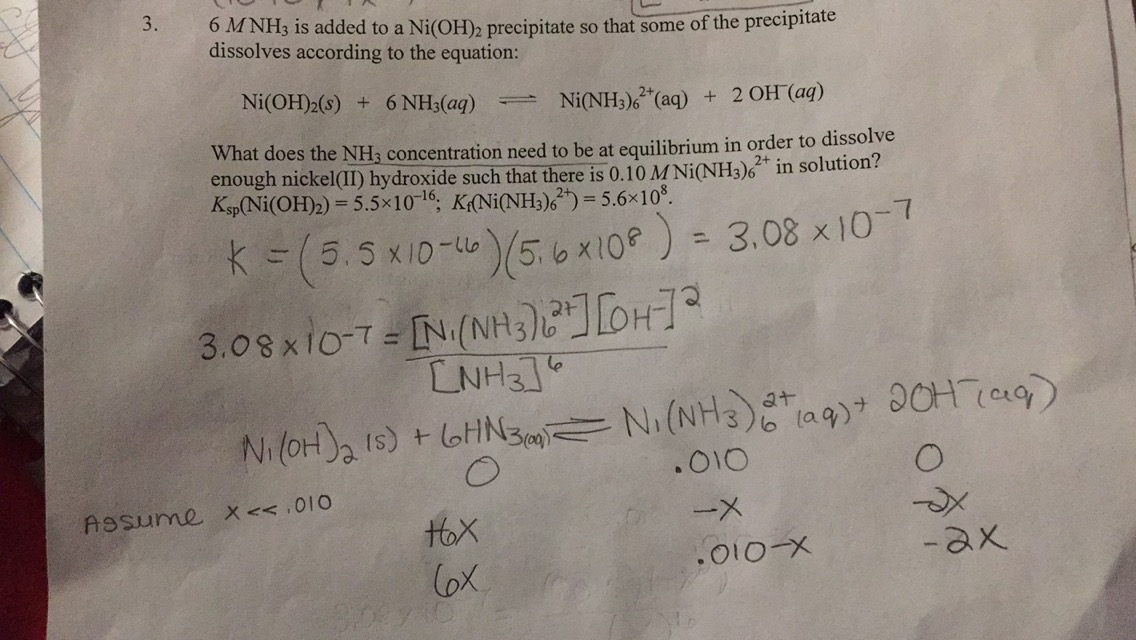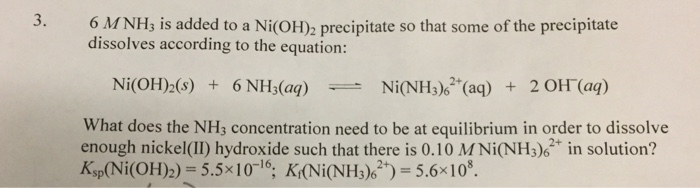Electrocatalytic ammonia oxidation over a nickel foam electrode: Role of Ni( OH)2(s)-NiOOH(s) nanocatalysts - ScienceDirect

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes

Electrocatalytic ammonia oxidation over a nickel foam electrode: Role of Ni( OH)2(s)-NiOOH(s) nanocatalysts - ScienceDirect

SEM images of various electrocatalysts synthesized in water and ammonia... | Download Scientific Diagram

OneClass: write a balanced net ionic equation for A. dissolving of Ni (OH)2 in nitric acid. B. Ni 2+ ...
![SOLVED: pleasee anwer asap (p) [NiOH]3+ reacts with NH3 and 1,2-diaminoethane(en) according to the reactions below: [Ni(OH]2+aq+6NH3aq)[NiNH]2+aq)+6HOI) 6=5.5108 [Ni(OHz)6]2+(aq)+3en (aq)=[Ni(en)]2+(aq)+6HO(I) 3=1.01018 i) Explain the differences in ... SOLVED: pleasee anwer asap (p) [NiOH]3+ reacts with NH3 and 1,2-diaminoethane(en) according to the reactions below: [Ni(OH]2+aq+6NH3aq)[NiNH]2+aq)+6HOI) 6=5.5108 [Ni(OHz)6]2+(aq)+3en (aq)=[Ni(en)]2+(aq)+6HO(I) 3=1.01018 i) Explain the differences in ...](https://cdn.numerade.com/ask_images/d9c7bbba11dd417bbdf667caa2ba53f9.jpg)
SOLVED: pleasee anwer asap (p) [NiOH]3+ reacts with NH3 and 1,2-diaminoethane(en) according to the reactions below: [Ni(OH]2+aq+6NH3aq)[NiNH]2+aq)+6HOI) 6=5.5108 [Ni(OHz)6]2+(aq)+3en (aq)=[Ni(en)]2+(aq)+6HO(I) 3=1.01018 i) Explain the differences in ...

Plots of log S of Ni(OH) 2 , Fe(OH) 2 , and Co(OH) 2 as a function of... | Download Scientific Diagram

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes

Stability-Enhanced α-Ni(OH)2 Pillared by Metaborate Anions for Pseudocapacitors | ACS Applied Materials & Interfaces

Stability-Enhanced α-Ni(OH)2 Pillared by Metaborate Anions for Pseudocapacitors | ACS Applied Materials & Interfaces

E735: Complex Ions and Precipitates – Nickel(II) compounds | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

Inorganics | Free Full-Text | Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate

Amongst the following, the total number of compounds soluble in concentrated NH3 solution is: Ag2CrO4,Cu(OH)2, PbSO3, Al(OH)3, Ni(OH)2, Zn3(PO4)2,BaSO4