
Ni(CO)4 Hybridisation , Geometry and Magnetic nature of Tetracarbonylnickel -coordination compounds - YouTube

Mechanism of Reppe's Nickel‐Catalyzed Ethyne Tetramerization to Cyclooctatetraene: A DFT Study - Straub - 2004 - Chemistry – A European Journal - Wiley Online Library
![Explain as to how the two complexes of nickel, [Ni(CN)4]2– and Ni(CO)4, have different structures but do not differ in their magnetic behaviour (Ni = 28). from Chemistry Coordination Compounds Class 12 Explain as to how the two complexes of nickel, [Ni(CN)4]2– and Ni(CO)4, have different structures but do not differ in their magnetic behaviour (Ni = 28). from Chemistry Coordination Compounds Class 12](https://www.zigya.com/application/zrc/images/qvar/CHEN12070326-1.png)
Explain as to how the two complexes of nickel, [Ni(CN)4]2– and Ni(CO)4, have different structures but do not differ in their magnetic behaviour (Ni = 28). from Chemistry Coordination Compounds Class 12
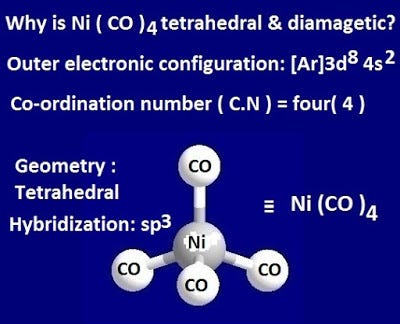
Why is Ni (CO) 4 tetrahedral and diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

Visible-Light-Driven Photocatalytic CO2 Reduction by a Ni(II) Complex Bearing a Bioinspired Tetradentate Ligand for Selective CO Production | Journal of the American Chemical Society

7. Komplexometria Analitika 13. C, 13. H osztály és 1219/6 modul tanfolyam részére 2010/ Komplexometria - ppt letölteni

A Two-Coordinate Nickel Imido Complex That Effects C−H Amination | Journal of the American Chemical Society

![Ni(CO)4] is a diamagnetic complex. Ni(CO)4] is a diamagnetic complex.](https://haygot.s3.amazonaws.com/questions/1305881_1317566_ans_5cb3a50509fd4ced94ab4354cf626da4.PNG)
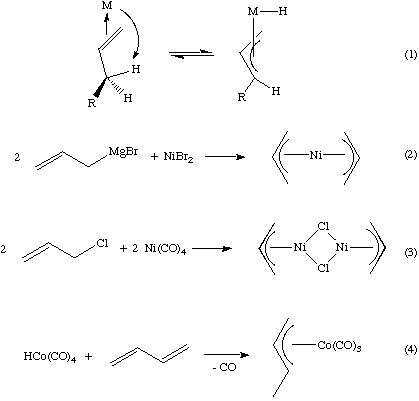

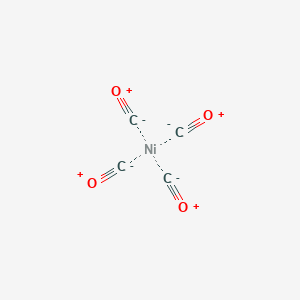



![Ni(CO)4] is a diamagnetic complex. Ni(CO)4] is a diamagnetic complex.](https://d1hhj0t1vdqi7c.cloudfront.net/v1/bmxMMm5hdGVNWk0=/sd/)
4.png)
![[Ni(CO)4] is a diamagnetic complex. [Ni(CO)4] is a diamagnetic complex.](https://i.ytimg.com/vi/nlL2nateMZM/maxresdefault.jpg)

![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-3.png)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](https://i.ytimg.com/vi/r_C4yyTUSjM/maxresdefault.jpg)



![Which is true in the case of [Ni(CO)4] complex? Which is true in the case of [Ni(CO)4] complex?](https://d10lpgp6xz60nq.cloudfront.net/physics_images/A2Z_CHM_XII_C09_E01_174_S01.png)